Nanoparticles
Particles in solution can be driven to translocate through nanopores/nanopipettes by a pressure difference and/or the application of a potential difference between internal and external electrodes. A translocating particle results in a resistive pulse (drop in the current between the two electrodes). For a conical geometry the resistive pulse is asymmetric, direction-dependent and gives information on many physical parameters of the particle; such as, the size, charge, deformability and shape of individual particles.
In the White group we have developed an understanding of the dynamics of particles travelling through pores, the forces involved, and the various ionic contributions to the shape of the resistive pulse. This understanding has enabled us to develop a multipass resistive pulse method, where we switch the pressure/voltage to repeatedly pass individual nanoparticles back and forth through the orifice of a conical nanopore/nanopipette. This leads to a precisely determined mean blocking current equating to sub-nanometer size resolution. We are currently exploring controlled delivery of single particles & molecules to electrochemical interfaces.
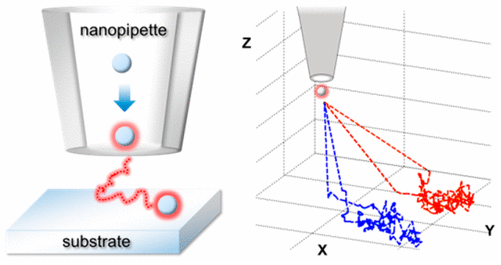
In addition, our group has developed a combined experimental/theoretical approach to investigate the dynamic physical and chemical interactions of individual suspended metal nanoparticles as they undergo electrochemical reactions at a microelectrode. To date, we have reported on the influence of Brownian motion on the electrochemical dissolution of single Ag nanoparticles as they collide with a polarized microelectrode. We explain the multimodal current vs. time responses by the Ag nanoparticle undergoing a series of discrete partial oxidation events as its random walk encounters the electrode surface.
Selected Publications:
Simultaneous Multipass Resistive-Pulse Sensing and Fluorescence Imaging of Liposomes
Schmeltzer, A.J., Peterson, E.M., Harris, J.M., Lathrop, D.K., German, S.R. and White, H.S.,
ACS nano, 2024, 18(9), 7241-7252.
Electrocatalytic Reduction of Benzyl Bromide during Single Ag Nanoparticle Collisions
Vitti, N.J. and White, H.S.,
Langmuir, 2024, 40(6), 3053-3062.
Critical Nucleus Size and Activation Energy of Ag Nucleation by Electrochemical Observation of Isolated Nucleation Events
Vitti, N.J., Majumdar, P. and White, H.S.,
Langmuir, 2023, 39(3), 1173-1180.
A High-Pressure System for Studying Oxygen Reduction During Pt Nanoparticle Collisions
Y. Zhang, D. A. Robinson, K. McKelvey, H. Ren, H. S. White, and M. A. Edwards
Journal of The Electrochemical Society, 2020, 167, 166507
Effect of Viscosity on the Collision Dynamics and Oxidation of Individual Ag Nanoparticles
D. A. Robinson, M. A. Edwards, Y. Liu, H. Ren. H. S. White
J. Phys. Chem. 2020, 124(16), 9068-9076
Electrochemical Synthesis of Individual Core@Shell and Hollow Ag/Ag2S Nanoparticles
D. A. Robinson, H. S. White
Nano Lett. 2019, 19(8), 5612-5619
Single Ag Nanoparticle Collisions within a Dual-Electrode Micro-Gap Cell
K. McKelvey, D. A. Robinson, N. J. Vitti, M. A. Edwards, H. S. White
Faraday Discuss., 2018, 210, 189-200
Effects of Instrumental Filters on Electrochemical Measurement of Single‐Nanoparticle Collision Dynamic
Donald Robinson, Martin A. Edwards, Hang Ren, and Henry S. White
ChemElectroChem, 2018, 5(20), 3059-3067
Collision and Oxidation of Silver Nanoparticles on a Gold Nanoband Electrode
F. Zhang, M. Edwards, R. Hao, H. S. White, B. Zhang
J. Phys. Chem. C, 2017, 121(42), 23564–23573
Three-Dimensional Super-resolution Imaging of Single Nanoparticles Delivered by Pipettes
Y. Yu, V. Sundaresan, S. Bandyopadhyay, Y. Zhang, M. A. Edwards, K. McKelvey, H. S. White, K. A. Willets.
ACS Nano, 2017, 11(10), 10529–10538
Observation of Multipeak Collision Behavior during the Electro-Oxidation of Single Ag Nanoparticles
S. M Oja, D. A. Robinson, N. J. Vitti, M. A. Edwards, Y. Liu, H. S. White, and B. Zhang
J. Am. Chem. Soc., 2017, 139(2), 708–718.
Resistive Pulse Delivery of Single Nanoparticles to Electrochemical Interfaces
K. McKelvey, M. A. Edwards, and H. S. White
J. Phys. Chem. Lett., 2016, 7, 3920–3924.
Resistive-Pulse Analysis of Nanoparticles
L. Luo, S. R. German, W. Lan, D. A. Holden, T. L. Mega, and H. S. White
Ann. Rev. of Anal. Chem. , 2014, 7, 513-535.
A high-speed multipass coulter counter with ultra-high resolution
M. A. Edwards, S. R. German, J. E. Dick, A. J. Bard, and H. S. White
ACS Nano, 2015, 9(12), 12274–12282.
Sizing Individual Au Nanoparticles in Solution with Sub-Nanometer Resolution
S. R. German, T. S. Hurd, H. S. White, and T. L. Mega
ACS Nano, 2015, 9(7), 7186-7194.
Effect of Surface Charge on the Resistive Pulse Waveshape during Particle Translocation through Glass Nanopores
W. Lan, C. Kubeil, J. Xiong, A. Bund, and H. S. White
J. Phys. Chem. C. , 2014, 118(5), 2726-2734.
Controlling Nanoparticle Dynamics in Conical Nanopores
S. R. German, L. Luo, H. S. White, and T. L. Mega
J. Phys. Chem C, 2013, 117(1), 703-711.
Nanopore Analysis of DNA
In collaboration with the Burrows group (University of Utah) we have a continuing interest in the use of biological nanopores to interrogate DNA structure. Much of our current work focuses on the use of the protein pore alpha-hemolysin (αHL) particularly for the identification of DNA damage. DNA damage is a significant issue in human cells, to take just one example de-purination of adenine occurs approximately 18,000 times in each cell per day. Unrepaired damage leads to mutations that in turn are responsible for the formation of cancers.
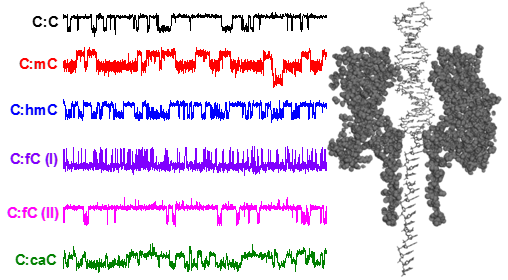
Under an applied potential, negatively charged DNA can be driven into the protein pore. Single-stranded DNA translocates through the pore with a characteristic translocation time, and attenuates the measured current as it moves through the pore. Both the translocation time and the current attenuation are characteristic of the DNA composition. We have found that the introduction of bulky damage sites in single-stranded DNA (e.g. Benoz[a]pyrene) will perturb translocation time and increase ion flux attenuation.
While ssDNA can pass through the smallest constriction in αHL (1.4 nm), dsDNA, which has a nominal diameter of 2.0 nm, cannot. However, it is possible to capture dsDNA inside the α-HL vestibule, and with an appropriate voltage (> 100 mV) the dsDNA duplex will unzip into its constituent single-stranded components. The residence time prior to unzipping is dependent on the composition of the duplex. We have recently discovered that structural modifications in dsDNA can be detected from the magnitude to which ion flow is attenuated during dsDNA residence inside the protein pore. The precise change in current is dependent on the position of the missing base in the sequence relative to the latch constriction of the α-HL protein pore, a newly discovered sensing zone in DNA (see figure below). We are currently exploring uses of this sensing zone for identifying and discriminating damage sites in dsDNA.
Select Publications:
γ-Hemolysin Nanopore Is Sensitive to Guanine-to-Inosine Substitutions in Double-Stranded DNA at the Single-Molecule Level
Cherie S. Tan, Aaron M. Fleming , Hang Ren , Cynthia J. Burrows, and Henry S. White
J. Am. Chem. Soc., 2018, 140 (43), pp 14224–14234.
Single-Molecule Titration in a Protein Nanoreactor Reveals the Protonation/Deprotonation Mechanism of a C:C Mismatch in DNA
Hang Ren, Cameron G. Cheyne, Aaron M. Fleming, Cythia J. Burrows, and Henry S. White
J. Am. Chem. Soc., 2018,140, 15, 5153-5160
Nanopore Analysis of the 5‑Guanidinohydantoin to Iminoallantoin Isomerization in Duplex DNA
T. Zeng, A. M. Fleming, Y. Ding, H. Ren, H. S. White, C. J. Burrows
J. Org. Chem., 2018, 83 (7), pp 3973–3978
Dynamics of a DNA Mismatch Site Held in Confinement Discriminate Epigenetic Modifications of Cytosine
R. P. Johnson, A. M. Fleming, R. T. Perera, C. J. Burrows, and H. S. White
J. Am. Chem. Soc., 2017, 139 (7), 2750–2756.
Interrogation of Base Pairing of the Spiroiminodihydantoin Diastereomers Using the α-Hemolysin Latch
T. Zeng, A. M. Fleming, Y. Ding, H. S. White, and C. J. Burrows
Biochemistry, 2017, 56 (11), 1596–1603.
Energetics of base flipping at a DNA mismatch site confined at the latch constriction of α-hemolysin
R. P. Johnson, R. T. Perera, A. M. Fleming, C. J. Burrows and H. S. White
Faraday Discuss., 2016, 193, 471-485.
Detection of benzo [a] pyrene-guanine adducts in single-stranded DNA using the α-hemolysin nanopore
R. T. Perera, A. M. Fleming, R. P. Johnson, C. J. Burrows, and H. S. White
Nanotechnology, 2015, 26(7), 074002.
Temperature and electrolyte optimization of the a-hemolysin latch sensing zone for detection of base modification in double-stranded DNA
R. P. Johnson, A. M. Fleming, Q. Jin, C. J. Burrows, and H. S. White
Biophys. J. , 2014, 107(4), 924-931.
Effect of an Electrolyte Cation on Detecting DNA Damage with the Latch Constriction of α-Hemolysin
R. P. Johnson, A. M. Fleming, C. J. Burrows, and H. S White
J. Phys. Chem. Lett., 2014, 5(21), 3781-3786.
Base-Excision Repair Activity of Uracil-DNA Glycosylase Monitored Using the Latch Zone of α-Hemolysin
Q. Jin, A. M. Fleming, R. P. Johnson, Y. Ding, C. J. Burrows, and H. S. White
J. Am. Chem. Soc., 2013, 135(51), 19347-19353.
Base-Excision Repair Activity of Uracil-DNA Glycosylase Monitored Using the Latch Zone of α-Hemolysin
Q. Jin, A. M. Fleming, R. P. Johnson, Y. Ding, C. J. Burrows, and H. S. White
J. Am. Chem. Soc., 2013, 135(51), 19347-19353.
Nanopore Physics
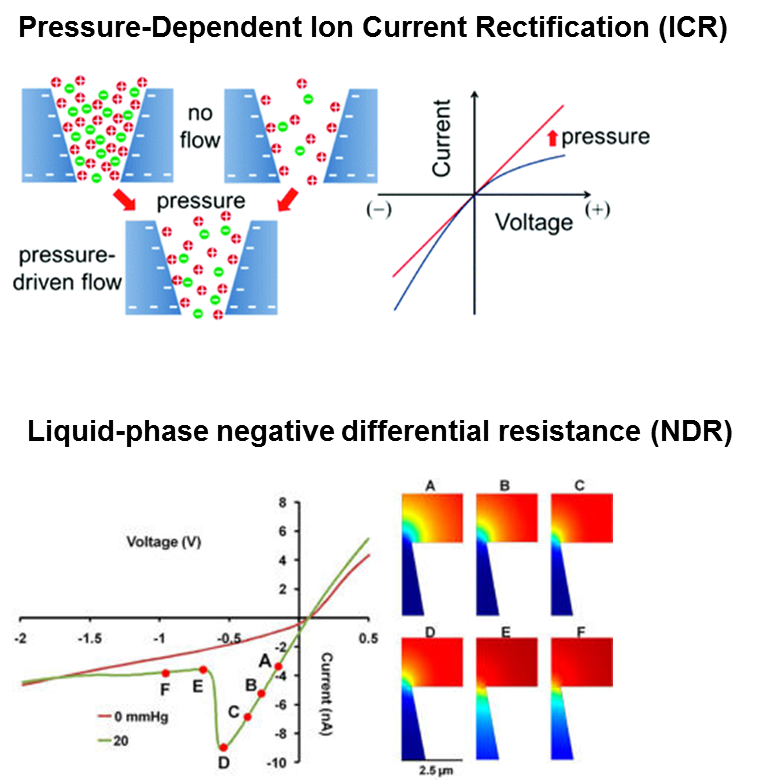
The ion transport in confined geometries is significantly different from bulk solutions. Ion Current rectification (ICR) is a phenomenon that arise due to the asymmetric charge distribution of the nanopores. We have solved Nernst-Planck, Poisson, and Navier-Stokes equations to simulate the ion transport phenomena in conical-shaped nanopores and demonstrated that the rectification is mainly due to a change in the ionic conductivity in the vicinity of the pore orifice. Negative differential resistance (NDR) is a technologically important electrical phenomenon in which electrical current decreases as an applied voltage is increased. NDR is also explained using both experimental and simulations. Moreover, we were able to show that the NDR switching potential is remarkably sensitive to the presence of Ca2+, suggesting possible applications in chemical sensing.
References:
Voltage-Rectified Current and Fluid Flow in Conical Nanopores
W. Lan, M. A. Edwards, L. Luo, R. T. Perera, X. Wu, C. R. Martin, and H. S. White
Acc. Chem. Res., 2016, 49(11), 2605–2613.
Negative Differential Electrolyte Resistance in a Solid-State Nanopore Resulting from Electroosmotic Flow Bistability
L. Lou, D. A. Holden, and H. S. White
ACS Nano., 2014, 8(3), 3023-3030.
Tunable Negative Differential Electrolyte Resistance in a Conical Nanopore in Glass
L. Luo, D. A. Holden, W. Lan, and H. S. White
ACS Nano, 2012, 6(7), 6507-6514.
Pressure-Dependent Ion Current Rectification in Conical-Shaped Glass Nanopores
W. Jin, D. A. Holden, and H. S. White
J. Am. Chem. Soc., 2011, 133(34), 13300-13303.
Ion Current Rectification at Nanopores in Glass Membranes.
H. S. White and A. Bund
Langmuir, 2008, 24(5), 2212-2218.
Transport in Thin Layer Batteries
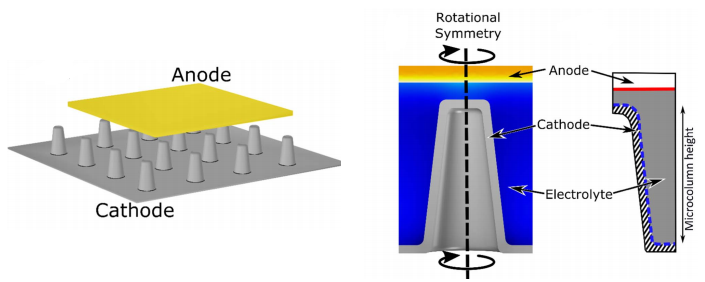
The need for smaller, more powerful, higher capacity batteries is ever increasing. This has resulted in the development of ultra-thin (ca. 10 nm) batteries in which the anode and cathode are separated by an ultra-thin electrolyte layer. At this length scale electrical double layers, that form at the electrodes (both the anode and cathode), begin to overlap and result in changes to the molecular transport of Li ions. We investigate this phenomenon theoretically using finite element simulations and experimentally using ultra-thin electrochemical cells.
Select Publications:
Microscale 2.5D Batteries
K. McKelvey, A. A. Talin, B. Dunn, and H. S. White
Journal of the Electrochemical Society, 2017, 164(12), A2500-A2503.
Fabrication, Testing and Simulation of All Solid State Three Dimensional Li-ion Batteries
A. A. Talin, D. Ruzmetov, A. Kolmakov, K. McKelvey, N. Ware, F. El Gabaly, B. S. Dunn, and H. S. White
ACS Appl. Mater. Interfaces, 2016, 8(47), 32385-32391.
Redox Cycling in Nanogap Electrochemical Cells. The Role of Electrostatics in Determining the Cell Response
Q. Chen, K. McKelvey, M. A. Edwards, and H. S. White
J. Phys. Chem. C, 2016, 120(31), 17251–17260.
Ion Transport within High Electric Fields in Nanogap Electrochemical Cells
J. Xiong, Q. Chen, M. A. Edwards, and H. S. White
ACS Nano., 2015, 9(8), 8520-8529.
Electron-Transfer Kinetics and Electric Double Layer Effects in Nanometer-Wide Thin-Layer Cells
L. Fan, Y. Liu, J. Xiong, H. S. White, and S. Chen
ACS Nano., 2014, 8(10), 10426-10436.
Nanobubbles
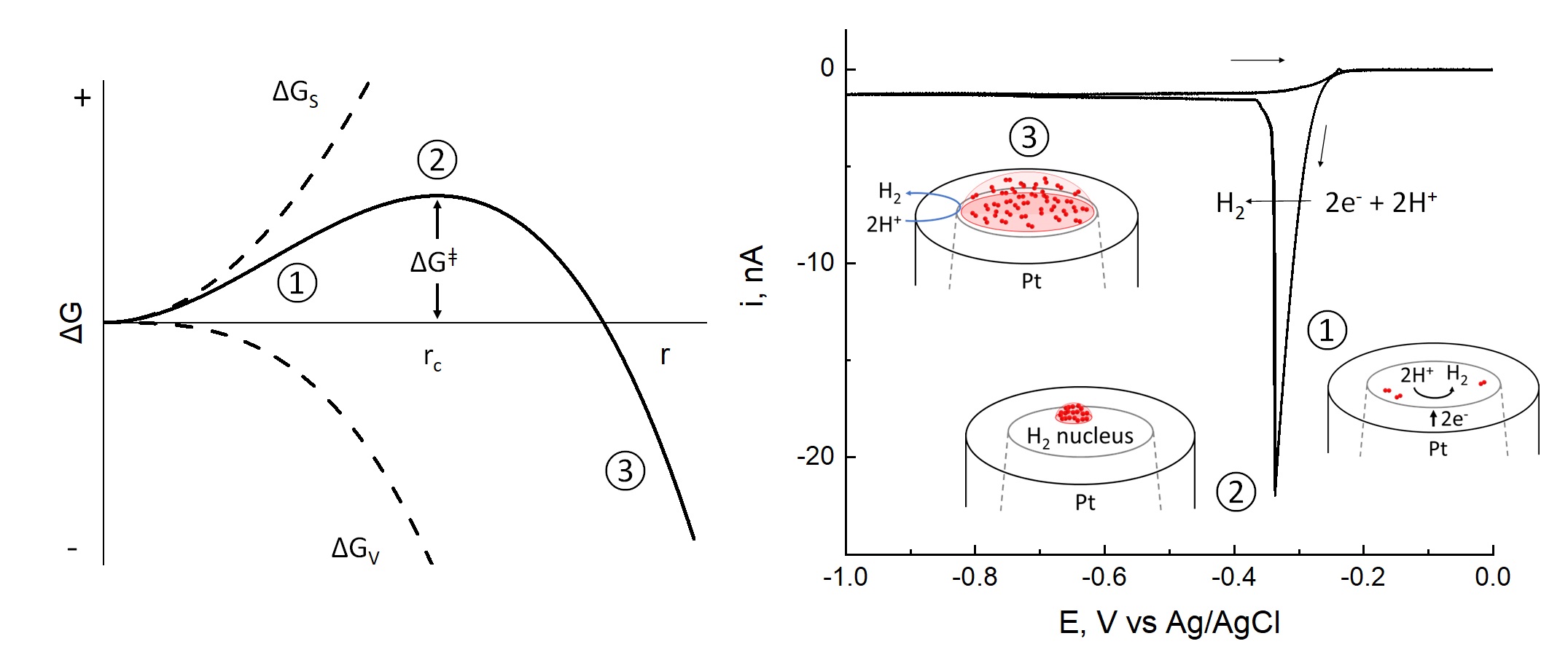
Gas nanobubbles formed at solid/liquid interfaces have received significant attention during the past decade due to their remarkable properties. We have developed an electrochemical approach for investigating the formation and properties of a single nanobubble of H2 with a radius between 5 and 50 nm. To create a H2 nanobubble, a Pt nanodisk electrode, shrouded in a glass sheath, is used to reduce H+ in a concentrated acid solution, creating a supersaturated solution of H2 adjacent to the electrode surface. As the electrode potential is scanned towards negative potentials, the current arising from H2 generation increases exponentially and then suddenly decreases to near background levels, signaling a liquid-to-gas phase transformation associated with the formation of a single nanobubble at the electrode surface. The nanobubble experiments reveal the nucleation mechanism of single nanobubble at the interface, as well as provide insight into the structure and chemical dynamics of electrochemical three-phase solid/liquid/gas boundaries.
Selected Publications:
Effect of Non-Specific Ion Adsorption and Parallel Faradaic Reactions on the Nucleation of H2 Nanobubbles
P. Majumdar, Y. Qiu and H. S. White
J. Electrochem. Soc. 2022, 169, 106515
Electroprecipitation of Nanometer-Thick Films of Ln(OH)3 [Ln = La, Ce, and Lu] at Pt Microelectrodes and Their Effect on Electron-Transfer Reactions
P. Majumdar, R. Gao, and H. S. White
Langmuir 2022, 38(26), 8125–8134
Effect of Nonuniform Mass Transport on Nanobubble Nucleation at Individual Pt Nanoparticles
N. S. Georgescu, D. A. Robinson, and H. S. White
J. Phys. Chem. C, 2021, 125(36), 19724–19732
Visualization and Quantification of Electrochemical H2 Bubble Nucleation at Pt, Au, and MoS2 Substrates
Y. Liu, C. Jin, Y. Liu, K. H. Ruiz, H. Ren, Y. Fan, H. S. White, and Q. Chen
ACS Sens. 2021, 6(2), 355–363
Electrochemical Generation of Individual Nanobubbles Comprising H2, D2, and HD
Y. Qiu, H. Ren, M. A. Edwards, R. Gao, K. Barman, and H. S. White
Langmuir, 2020, 36(22) 6073–6078
Nitrogen Bubbles at Pt Nanoelectrodes in Non-Aqueous Medium-Oscillating Behavior and Geometry of Critical Nuclei
Q. Chen, Y. Liu, M. A. Edwards, Y. Liu, and H. S. White
Anal. Chem. 2020. 92(9), 6408-6414
Electrochemically Controlled Nucleation of Single CO2 Nanobubbles via Formate Oxidation at Pt Nanoelectrodes
H. Ren, M. A. Edwards, Y. Wang, H. S. White
J. Phys. Chem. Lett.2020, 11(4), 1291-1296.
Voltammetric Determination of the Stochastic Formation Rate and Geometry of Individual H2,N2, and O2Bubble Nuclei
M. A. Edwards, H. S. White, H. Ren
ACS Nano. 2019, 13(6), 6330-6340
The Nucleation Rate of Single O2 Nanobubbles at Pt Electrodes
Álvaro Moreno Soto, Sean R. German, Hang Ren, Devaraj van der Meer, Detlef Lohse, Martin A. Edwards, and Henry S. White
Langmuir, 2018,34(25), 7309–7318
Critical Nuclei Size, Rate, and Activation Energy of H2 Gas Nucleation
Sean R. German, Martin A. Edwards, Hang Ren, and Henry S. White
J. Am. Chem. Soc., 2018, 140(11), 4047–4053
Electrochemical Generation of Individual O2 Nanobubbles via H2O2 Oxidation
H. Ren, S. R. German, M. A. Edwards, Q. Chen, and H. S. White
J. Phys. Chem. Lett., 2017, 8, 2450-2454.
The dynamic steady state of an electrochemically generated nanobubble
Y. Liu, M. A. Edwards, S. R. German, Q. Chen, and H. S. White
Langmuir, 2017, 33(8), 1845–1853.
Laplace Pressure of Individual H2 Nanobubbles from Pressure-Addition Electrochemistry
S. R. German, M. A. Edwards, Q. Chen, and H. S. White
Nano Lett., 2016, 16(10), 6691–6694.
Electrochemical Nucleation of Stable N2 Nanobubbles at Pt Nanoelectrodes
Q. Chen, H. S. Wiedenroth, S. R. German, and H. S. White
J. Am. Chem. Soc., 2015, 137(37), 12064–12069.
Electrochemical generation of a hydrogen bubble at a recessed platinum nanopore electrode
Q. Chen, L. Luo, and H. S. White
Langmuir, 2015, 31(15), 4573-4581.
Electrochemical Measurements of Single H2 Nanobubble Nucleation and Stability at Pt Nanoelectrodes
Q. Chen, L. Luo, H. Faraji, S. W. Feldberg, and H. S. White
J. Phys. Chem. Lett. , 2014, 5(20), 3539-3544.
Electrogeneration of Single Nanobubbles at Sub-50-nm-Radius Platinum Nanodisk Electrodes
L. Luo and H. S. White
Langmuir, 2013, 29(35), 11169-11175.
Modelling Experimental Systems
For the systems investigated in the White group the experimentally measured quantity is most typically the current, or a statistical measure derived from it, whereas our interest usually lies in the underlying physical and chemical phenomena. Interpretation of our data is typically underpinned by a theoretical model of the system, which may be an analytical expression or a numerical calculation, relating the measured quantity to one or more physical quantities. Often no model of the system we are studying exist and we develop one. Typical problems include, for instance, relating electron-transfer rates with the local electrostatic potential distribution, computing the forces of a nanoparticle and its resulting velocity, or studying the effect of ion transport in confined spaces on the discharge rate of a 3D battery.
As well as allowing us to go from an experimentally measured quantity to a physical quantity, models also allow us to ask “what if…?” questions of our experiments. We can effectively prototype experiments to deduce whether we are likely to be sensitive to a particular effect. This also allows us to investigate the propagation of uncertainties in our work.
We use the commercial finite element package Comsol Multiphysics to build and solve numerical models that describe multiple interacting physical processes; such as electric fields, fluid flow, and the transport of species in solution though a combination of diffusion, electromigration and convective transport. Below are select publications showcasing a number of theoretical models that the White group has developed.
Selected Publications:
Electric Potential-Driven Acid/Base Chemistry: Kinetics of Electrochemical Interfacial Proton Transfer and Transport
Pendergast, A.D., Levey, K.J., Macpherson, J.V., Edwards, M.A. and White, H.S.,
The Journal of Physical Chemistry C, 2024, 128(17), 7127-7136.
Double-Layer Inhibition of Peroxydisulfate Reduction at Mercury Ultramicroelectrodes. A Quantitative Analysis of the Frumkin Effect Including Molecular Transport and Long-Range Electron Transfer
Pendergast, A.D. and White, H.S.,
The Journal of Physical Chemistry C, 2023, 127(23), 11283-11297
Simulation of the cyclic voltammetric response of an outer-sphere redox species with inclusion of electrical double layer structure and ohmic potential drop
Levey, K.J., Edwards, M.A., White, H.S. and Macpherson, J.V.,
Physical Chemistry Chemical Physics, 2023, 25(11), 7832-7846
Finite Element Modeling of the Combined Faradaic and Electrostatic Contributions to the Voltammetric Response of Monolayer Redox Films
K. J. Levey, M. A. Edwards, H. S. White, and J. V. Macpherson
Anal. Chem. 2022, 94(37), 12673-12682
Nanopore Opening and Flat and Nano-Tip Conical Electrodes during Vesicle Impact Electrochemcial Cytometry
X. Li, Lin Ren, J. Dunevall, D. Ye, H. S. White, M. A. Edwards, and A. G. Ewing
ACS Nano, 2018, 12(3), 3010–3019
Collision Dynamics During the Electrooxidation of Individual Silver Nanoparticles
D. A Robinson, Y. Liu, M. A. Edwards, N. J. Vitti, S. M. Oja, B. Zhang, H. S. White
J. Am. Chem. Soc., 2017,139(46), 16923–16931
Voltage-Rectified Current and Fluid Flow in Conical Nanopores
W. Lan, M. A. Edwards, L. Luo, R. T. Perera, X. Wu, C. R. Martin, and H. S. White
Acc. Chem. Res., 2016,49(11), 2605–2613.
Ion Transport within High Electric Fields in Nanogap Electrochemical Cells
J. Xiong, Q. Chen, M. A. Edwards, and H. S. White
ACS Nano, 2015, 9(8), 8520-8529.
Effect of Surface Charge on the Resistive Pulse Waveshape during Particle Translocation through Glass Nanopores
W. Lan, C. Kubeil, J. Xiong, A. Bund, and H. S. White
J. Phys. Chem. C. , 2014, 118(5), 2726-2734.
Negative Differential Electrolyte Resistance in a Solid-State Nanopore Resulting from Electroosmotic Flow Bistability
L. Lou, D. A. Holden, and H. S. White
ACS Nano. , 2014, 8(3), 3023-3030.
Electrogeneration of Single Nanobubbles at Sub-50-nm-Radius Platinum Nanodisk Electrodes
L. Luo and H. S. White
Langmuir, 2013, 29(35), 11169-11175.
Synthetic Organic Electrochemistry
Electrochemistry represents a powerful tool within synthetic organic chemistry. Electrodes can be used to generate highly reactive and short-lived species, which are otherwise unfeasible to create/use in synthetic organic chemistry. Moreover, the current produced at these electrodes can be used to monitor the presence of electroactive species and probing reaction mechanisms.
As a member of the NSF funded Center for Chemical Innovation: Center for Synthetic Organic Electrochemistry, the White group is at the forefront of developing a fundamental understanding of green, safe, and economic electrochemical reactions. In doing this, we employ a range of electrochemical techniques, e.g., voltammetry, scanning electrochemical microscopy (SECM), enabling the characterization of kinetically fast reactions over short time-scales.
References:
Investigation of the Electrocatalytic Reduction of Peroxydisulfate Using Scanning Electrochemical Microscopy
Hosseini, S., Solymosi, G.T. and White, H.S.,
Anal. Chem.2024, 96(21), 8424–8431
Unraveling hydrogen atom transfer mechanisms with voltammetry: oxidative formation and reactivity of cobalt hydride
Boucher, D.G., Pendergast, A.D., Wu, X., Nguyen, Z.A., Jadhav, R.G., Lin, S., White, H.S. and Minteer, S.D.,
Journal of the American Chemical Society, 2023, 145(32), 17665-17677.
Electroorganic synthesis in aqueous solution via generation of strongly oxidizing and reducing intermediates
Hosseini, S., Beeler, J.A., Sanford, M.S. and White, H.S.,
Faraday Discussions, 2023, 247, 192-205.
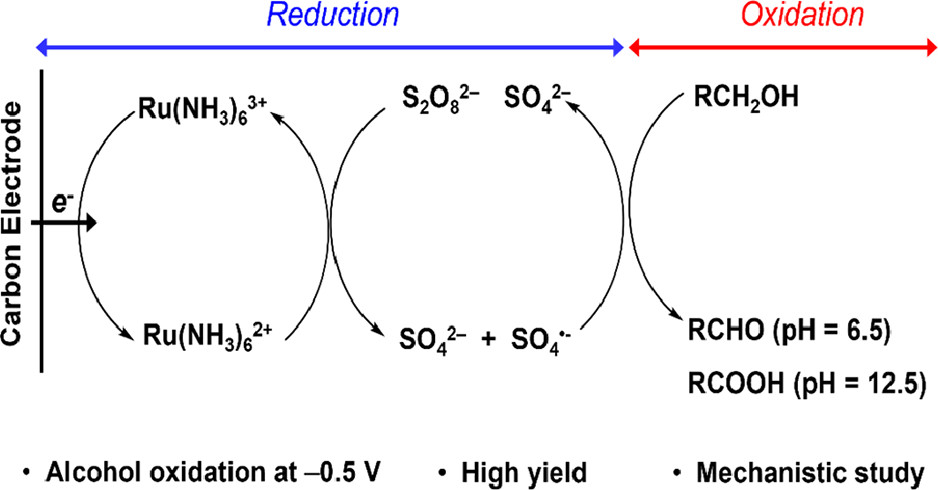
Oxidation by Reduction: Efficient and Selective Oxidation of Alcohols by the Electrocatalytic Reduction of Peroxydisulfate
S. Hosseini, J. N. Janusz, M. Tanwar, A. D. Pendergast, M. Neurock, and H. S. White
J. Am. Chem. Soc. 2022, 144(46), 21103–21115
Electrochemical Reduction of [Ni(Mebpy)3]2+. Elucidation of the Redox Mechanism by Cyclic Voltammetry and Steady-State Voltammetry in Low Ionic Strength Solutions.
K. Barman, M. A. Edwards, D. P. Hickey, C. Sandford, Y. Qiu, R. Gao, S. D. Minteer, H. S. White
ChemElectroChem. 2020,7(6), 1473-1479
Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications
Y. Kawamata, J. C. Vantourout, D. P. Hickey, P. Bai, L. Chen, Q. Hou, W. Qiao, K. Barman, M. A. Edwards, A. F. Garrido-Castro, J. N. deGruyter, H. Nakamura, K. W. Knouse, C. Qin, K. J. Clay, D. Bao, C. Li, J. T. Starr, C. Garcia-Irizarry, N. Sach, H. S. White, M. Neurock, S. D. Minteer, and P. S. Baran
J. Am. Chem. Soc., 2019, 141(15), 6392-6402.
