| Molecular recognition and protein-RNA complex formation | |
© The Royal Society of Chemistry 2021 |
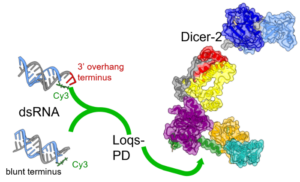
Using time-resolved photoluminescence (TRPL) to measure light emitted by probe labeling RNA substrates, it is possible to detect the signatures of binding as they alter the probe's photophysics (e.g., fluorescence lifetime, transient anisotropy). In this way, one can differentiate between different modes of molecular recognition employed by large, multidomain proteins (such as Dicer-2, left) |
© The Royal Society of Chemistry 2024 |
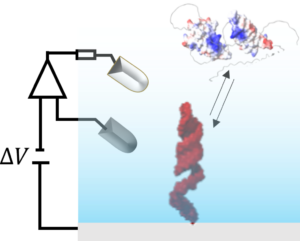
Combining expertise in materials science and physical chemistry, we devised an experimental platform that incorporates mid-IR plasmon reflectivity and time-resolved fluorescence measurements at the surface of a working electrode. Controlling the local environment of biomolecules allows us to study their conformations and binding properties. |
| Solvation and kinetics in polymorph selection | |
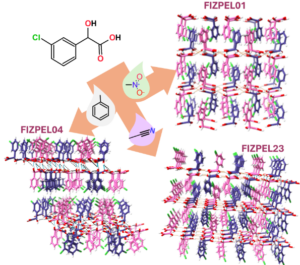 |
Predicting the crystallization outcomes of flexible organic molecules is a challenging task, especially when different crystal structures (polymorphs) are possible. Together with the Gruenwald and Looper groups at U. Utah, we seek to determine the role of solution species on the kinetics of polymorph selection and stability. |
| Photochemistry in solution | |
 |
|
© 2024 American Chemical Society
Correlative imaging relies on tags that can generate contrast in more than one imaging modality -- such as light microscopy and electron microscopy. Flavoproteins are widely used to this end. Together with the Hammond group au U. Utah. we seek to understand the photochemical properties that sustain the localized growth of polymeric contrast agents, expanding the palette of sensitizers, polymer building blocks, and stains to enable new applications.
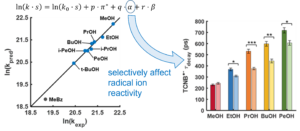
© 2022 American Chemical Society
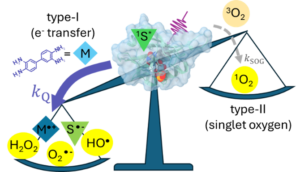
Using ultrafast UV photochemistry, we identified transient radical ion species that efficiently equilibrate with their environment and then participate in a chemical transformation from an electronic ground state whose reactivity can be altered by solute-solvent interactions. Solvent-mediated reactive pathways are an important degradation channel for radical ions used in energy conversion and storage materials, and we are exploring ways to tune their local environment to reduce the degradation of redox-active species within complex electrolytes.
| Dynamic environments in electrochemistry | |
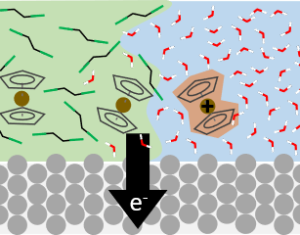 |
Electrochemical processes occur within a complex environment involving electrode surfaces, solvent molecules, ions, and free and surface-bound species. We are particularly interested in reactions where large solvation shell changes (e.g., phase transfer) occur concurrently with redox chemistry. This exciting collaboraton with the White group at U. Utah and the Voth group at U. Chicago aims to understand the molecular-scale determinants of these important class of reactions. |
We gratefully acknowledge financial support of our research by the National Science Foundation (awards 2123516 and 2300863), the W. M. Keck Foundation, the Alfred P. Sloan Foundation, and the University of Utah.
Contact us to learn more about our work at the interface of spectroscopy and materials chemistry.
