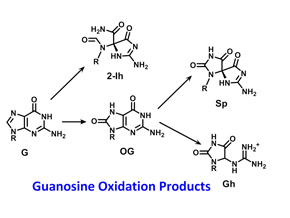
The heterocyclic bases of DNA and RNA are subject to chemical modification, either accidentally or on purpose. For example, endogenous oxidative stress or external agents such as radiation lead to oxidation of the guanine base in both DNA and RNA. In DNA, this process is traditionally thought to be mutagenic, and an accumulation of mutations can lead to cancer. We recently demonstrated that oxidized bases can also be epigenetic, particularly in G-rich gene promoters where they impact gene expression via the base excision repair pathway.
This research program has led to the following activities and goals in the lab:
- Understanding the mechanistic chemistry of guanine oxidation using free radical oxidants that are relevant to cellular chemistry
- Examining the reactivity and folding dynamics of G-rich sequences that form G-quadruplexes (and their complementary i-motif structures)
- Developing sequencing protocols, both nanopore and Illumina-based, for finding oxidized bases in DNA and RNA
- Using cell-based assays to understand the impact of DNA modifications on G-expression in normal vs. cancer cells
- Analyzing DNA repair activity on oxidized bases and abasic sites
- Determining the interplay of chemical modifications in DNA (genomic) and RNA (mRNA and viral RNA) with structural motifs such as G-quadruplexes. Recently, this has involved 8-oxoG in DNA and RNA, and N6-methyladenosine, pseudouridine and inosine in RNA.
Cynthia Burrows is a member of the Nano Institute of Utah.
Cynthia J. Burrows
Distinguished Professor
Thatcher Presidential Endowed Chair of Biological Chemistry
